Match each specific solution with the principal intermolecular force exhibited. – Understanding the interplay between specific solutions and their principal intermolecular forces is crucial in chemistry and various scientific disciplines. Intermolecular forces govern the behavior and properties of substances, influencing their solubility, reactivity, and physical characteristics. This article delves into the concept of matching specific solutions with their predominant intermolecular forces, providing a comprehensive overview of the topic.
The following paragraphs explore the different types of intermolecular forces, their impact on solutions, and practical applications of matching solutions with appropriate forces. By examining real-world examples and considering advanced factors such as temperature and pressure, we gain a deeper understanding of the significance and complexities of this fundamental concept.
Intermolecular Forces
Intermolecular forces are the forces that act between molecules. They are weaker than the intramolecular forces that hold atoms together within a molecule, but they are strong enough to affect the physical properties of a substance.
There are three main types of intermolecular forces:
- Hydrogen bondingis the strongest type of intermolecular force. It occurs when a hydrogen atom is bonded to a highly electronegative atom, such as oxygen, nitrogen, or fluorine. The electronegative atom attracts the electrons in the hydrogen bond, creating a partial positive charge on the hydrogen atom and a partial negative charge on the electronegative atom.
These partial charges attract each other, forming a hydrogen bond.
- Dipole-dipole forcesoccur when a molecule has a permanent dipole moment. This means that the molecule has a positive end and a negative end. The positive end of one molecule attracts the negative end of another molecule, forming a dipole-dipole force.
- London dispersion forcesare the weakest type of intermolecular force. They occur when electrons in a molecule are not evenly distributed. This creates a temporary dipole moment, which can attract the electrons in another molecule, forming a London dispersion force.
Specific Solutions and Intermolecular Forces
The type of intermolecular force that a substance exhibits depends on the structure of the molecule. The following table lists some common substances and the intermolecular forces that they exhibit:
| Solution | Solvent | Intermolecular Force | Explanation |
|---|---|---|---|
| Water | Water | Hydrogen bonding | Water molecules have a permanent dipole moment due to the electronegativity difference between oxygen and hydrogen. This allows water molecules to form hydrogen bonds with each other, which is responsible for the high boiling point of water. |
| Ethanol | Water | Hydrogen bonding | Ethanol molecules also have a permanent dipole moment due to the electronegativity difference between oxygen and hydrogen. This allows ethanol molecules to form hydrogen bonds with water molecules, which is why ethanol is miscible with water. |
| Hexane | Hexane | London dispersion forces | Hexane molecules are nonpolar, so they do not have a permanent dipole moment. However, the electrons in hexane molecules are not evenly distributed, which creates a temporary dipole moment. This allows hexane molecules to form London dispersion forces with each other. |
| Sodium chloride | Water | Ion-dipole forces | Sodium chloride is an ionic compound, which means that it is composed of positively charged sodium ions and negatively charged chloride ions. When sodium chloride is dissolved in water, the water molecules surround the ions and form ion-dipole forces. |
| Sucrose | Water | Hydrogen bonding and dipole-dipole forces | Sucrose molecules have a permanent dipole moment due to the electronegativity difference between oxygen and hydrogen. They also have hydroxyl groups, which can form hydrogen bonds with water molecules. This combination of intermolecular forces makes sucrose soluble in water. |
Matching Solutions and Forces: Match Each Specific Solution With The Principal Intermolecular Force Exhibited.
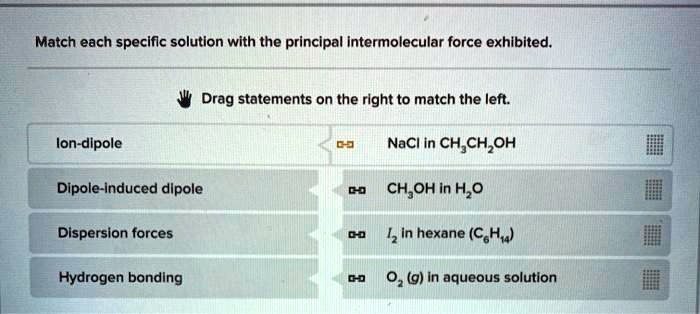
To match a specific solution with its principal intermolecular force, you can use the following steps:
- Identify the type of solute.Is the solute an ionic compound, a polar molecule, or a nonpolar molecule?
- Identify the type of solvent.Is the solvent water, a polar organic solvent, or a nonpolar organic solvent?
- Use the table in the previous section to match the type of solute with the type of solvent.The table will tell you the principal intermolecular force that is responsible for the solubility of the solute in the solvent.
Examples and Applications
Matching solutions and forces is important in a variety of fields, including chemistry, biology, and materials science.
- In chemistry, matching solutions and forces is important for understanding the solubility of different substances. This knowledge can be used to design new materials and to develop new chemical processes.
- In biology, matching solutions and forces is important for understanding the interactions between different molecules in living organisms. This knowledge can be used to develop new drugs and to treat diseases.
- In materials science, matching solutions and forces is important for understanding the properties of different materials. This knowledge can be used to design new materials with specific properties.
Using the wrong solution or force in a given situation can have a variety of consequences. For example, using a nonpolar solvent to dissolve an ionic compound will not work because the nonpolar solvent will not be able to form ion-dipole forces with the ionic compound.
This will result in the ionic compound precipitating out of solution.
Advanced Considerations

The strength of intermolecular forces can be affected by temperature and pressure. As temperature increases, the strength of intermolecular forces decreases. This is because the molecules are moving faster at higher temperatures, which makes it more difficult for them to form intermolecular forces.
Pressure can also affect the strength of intermolecular forces. As pressure increases, the molecules are forced closer together, which makes it easier for them to form intermolecular forces. This is why gases are more soluble in liquids at high pressures.
The matching process is not always straightforward. There are some exceptions to the rules that we have discussed. For example, some polar molecules are not soluble in water. This is because the polar molecules can form stronger intermolecular forces with themselves than they can with water molecules.
Detailed FAQs
What are the different types of intermolecular forces?
The main types of intermolecular forces are hydrogen bonding, dipole-dipole interactions, London dispersion forces, and ion-dipole interactions.
How do intermolecular forces affect the properties of solutions?
Intermolecular forces influence the solubility, viscosity, boiling point, and other physical properties of solutions.
Why is it important to match specific solutions with the appropriate intermolecular forces?
Matching solutions with suitable intermolecular forces is crucial for optimizing chemical reactions, designing materials with specific properties, and understanding the behavior of substances in different environments.